In 2007, a research team spread across 23 sites worldwide conducted the first large-scale study of TMS for depression. The researchers found that participants who received rTMS showed significant improvement in their depression, compared to a group of participants who received a “sham” TMS treatment (which mimics the experience of receiving TMS without actually delivering TMS to the participant, eliminating concerns that positive improvements in depression could be caused by the placebo effect).4 Within a year of the landmark study, the Food and Drug Administration (FDA) approved TMS as a safe, effective therapy for treatment-resistant depression.
After the treatment received FDA approval, the field of neuroscience saw an explosion of interest and research about TMS. Although the 2007 study had already determined that TMS is an effective treatment for depression, other researchers began to conduct their own studies to confirm the findings of the prior research and to better understand this innovative new treatment. Importantly, the researchers understood that scientific evidence is strongest when many different studies show similar results using the same measurement methods. Accordingly, most of the follow-up studies assessed the effectiveness of TMS by using well-known, commonly accepted assessments of depression symptoms, including the Montgomery-Asberg Depression Rating Scale (MADRS) and the Hamilton Depression Rating Scale (HAMD). Researchers administered these assessments to the study participants before, during, and after TMS treatment to track improvements in their depression symptoms over time, indicating the effectiveness of the treatment.
Among these new studies was a large clinical trial funded by the National Institute of Mental Health (NIMH) in 2010,5 which found that:
- Participants who received rTMS were 4.2 times more likely to achieve remission than those who received a sham treatment.
- About 15% of patients responded at least somewhat positively (that is, their depression improved), and another 15% achieved full remission from depression.
- The remission rate increased to 30% in a follow-up study that delivered rTMS to all of the initial participants, including those who had originally received the sham treatment.
Once there was a solid foundation of evidence for the effectiveness of TMS for depression, other researchers began to review and analyze findings from the existing clinical trials. This “study of studies,” allowed the researchers evaluate all of the existing high-quality evidence and develop recommendations that account for a diverse pool of evidence.
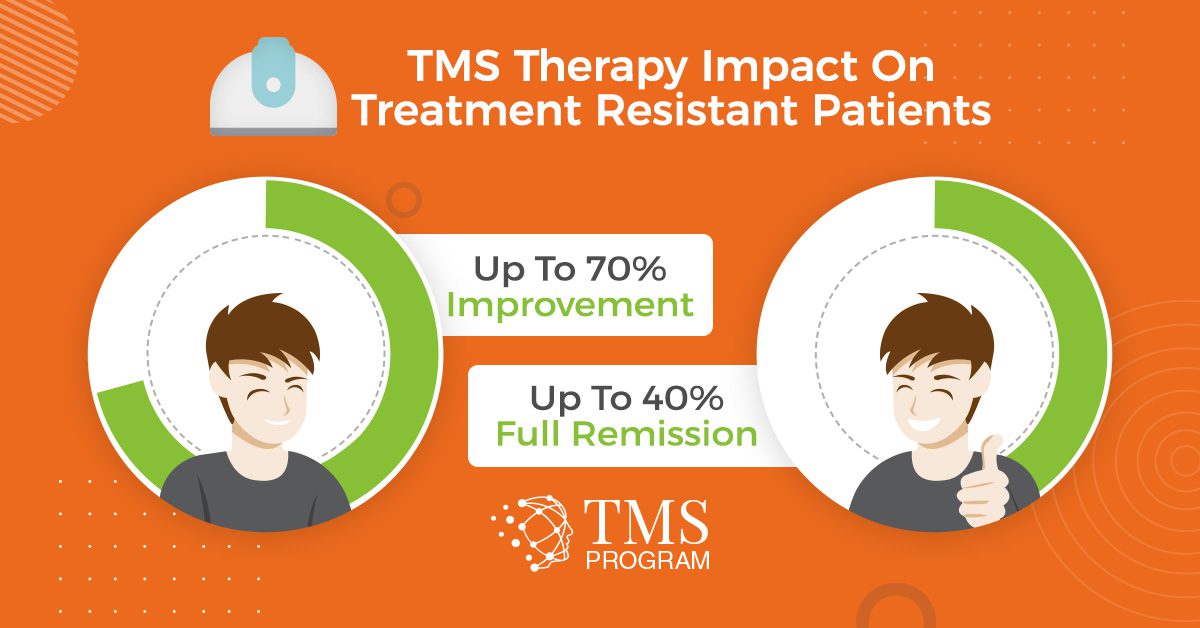
In 2013, one of these comprehensive reviews compiled data from eight previous clinical trials on rTMS for depression.6 The researchers found that, across these eight studies:
- Participants underwent 12 to 13 TMS sessions on average.
- 38.2% of participants who received rTMS showed some positive improvement, and an additional 34.6% experienced full remission.
- Trials that used more than 1,200 magnetic pulses during the TMS treatment course showed higher response rates, indicating a potential benchmark pulse dosage for optimal treatment success.
Based on the strength of these findings, BrainsWay conducted a large clinical trial in 2015 using BrainsWay TMS technology,7 finding that:
- Within five weeks of receiving regular rTMS treatment with the BrainsWay tool, patients showed an average 6.39 point increase on the HAMD—nearly double the 3.11 point increase among comparison patients who received the sham treatment.
- By the end of the study at week 16, 44.3% of participants receiving rTMS responded positively, while an additional 31.8% experienced full remission.
- The participants maintained their improvements for at least three months after completing TMS.
In 2016, a team of scientists from the Clinical TMS Society contributed their expertise by conducting another review of the published evidence on TMS.8 After analyzing the large number of TMS clinical trials published in peer-reviewed journals, they again confirmed that TMS is a safe, effective therapy for treatment-resistant depression. Notably, the Clinical TMS Society also addressed some concerns about the long-term sustainability of symptom improvement in the months and years following TMS treatment. In addition to the evidence for sustainable symptom improvement from the 2015 BrainsWay trial,7 the Clinical TMS Society highlighted results from other important clinical studies:
- In the 2010 NIMH study, only 5 of 60 patients who achieved remission experienced a relapse within 3 months of treatment, and 4 of these 5 patients regained remission of depression after subsequent TMS treatments.5
- A study found that after four weeks of daily TMS treatment sessions, 18 weeks of once-to-twice weekly follow-up treatment with TMS improved and preserved the effects of antidepressant medications among people whose depression was treatment-resistant.9
- A 2015 Neuronetics study found that patients whose depression improved after a full course of TMS were able to maintain their progress by receiving one TMS session per month following the end of the standard treatment course. A high proportion (78%) of participants who initially had a positive response to TMS would respond positively again to future TMS treatments, if needed.10
Given that the vast majority of participants maintain their improvements after completing TMS treatment (or are easily able to re-gain their improvements with follow-up treatment), the Clinical TMS Society concluded that TMS is an effective long-term therapeutic option for depression. Researchers today are working to develop a standardized protocol for maintaining the positive progress associated with TMS, ensuring that individuals experience permanent improvement or remission.11
The remarkable success of TMS for treatment-resistant depression has inspired researchers to investigate the technique for other mental health disorders. In 2018, the FDA approved TMS as a safe, effective therapy for treatment-resistant obsessive compulsive disorder (OCD) based on results from a clinical trial that found that 38.1% of patients with treatment-resistant OCD who received TMS showed at least a 30% reduction in their Yale-Brown Obsessive Compulsive Scale score, indicating positive response to the treatment.12
As scientists and innovators continue their work to pioneer exciting new advances in TMS technology, they recognize that the consensus among experts is clear: there is strong, extensive evidence to indicate that TMS is an effective, convenient, non-invasive treatment option for people whose depression does not respond to traditional antidepressant medications. Rapid innovations in TMS remain a testament to the science community’s commitment to alleviating the burden of depression among all those who suffer from the condition, offering new hope where traditional therapies have failed.
Citations
1 How Does TMS Work. Butler Hospital. Care New England Health System. (2020). Retrieved from http://www.butler.org/butler-ri/programs/outpatient/how-does-tms-work.cfm
2 Al-Harbi KS. (2012). Treatment-resistant depression: Therapeutic trends, challenges, and future directions. Patient Preference and Adherence, 2012(6):369-388.
3 Berlim MT, Van den Eynde F, Tovar-Perdomo S, et al. (2015). Augmenting antidepressants with deep transcranial magnetic stimulation (dTMS) in treatment-resistant major depression. World Journal of Biological Psychiatry, 15(7):570-578.
4 O’Reardon JP, Solvason HB, Janicak PG, et al. (2007). Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11):1208-1216.
5 George MS, Lisanby SH, Avery D, et al. (2010). Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder. Archives of General Psychiatry, 67(5):507-516.
6 Berlim MT, Van den Eynde F, & Daskalakis ZJ. (2013). Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: A meta-analysis of randomized, double-blind, and sham-controlled trials. Neuropsychopharmacology, 38:543-551.
7 Levkovitz Y, Isserles M, Padberg F, et al. (2015). Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry, 14(1):64-73.
8 Perera T, George MS, Grammer G, et al. (2016). The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulation, 9:336-346.
9 Harel EV, Rabany L, Deutsch L, et al. (2012). H-coil repetitive transcranial magnetic stimulation for treatment resistant major depression disorder: An 18-week continuation safety and feasibility study. World Journal of Biological Psychiatry, 15(4):298-306.
10 Philip NS, Dunner DL, Dowd SM, et al. (2016). Can medication free, treatment-resistant, depressed patients who initially respond to TMS be maintained off medications? A prospective, 12-month multisite randomized pilot study. Brain Stimulation, 9(2):251-257.
11 Baeken C, Brem AK, Arns M, et al. (2019). Repetitive transcranial magnetic stimulation treatment for depressive disorders: Current knowledge and future directions. Current Opinions in Psychiatry, 32:409-415.
12 Carmi L, Tendler A, Bystristky A, et al. (2019). Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: A prospective multicenter randomized double-blind placebo-controlled trial. American Journal of Psychiatry, 176(11):931-938.